RxODE
Overview
RxODE is an R package for solving and simulating from ode-based models. These models are convert the RxODE mini-language to C and create a compiled dll for fast solving. ODE solving using RxODE has a few key parts:
-
RxODE()which creates the C code for fast ODE solving based on a simple syntax related to Leibnitz notation. - The event data, which can be:
- a
NONMEMordeSolvecompatible data frame, or - created with
et()orEventTable()for easy simulation of events - The data frame can be augmented by adding time-varying or adding individual covariates (
iCov=as needed)
- a
-
rxSolve()which solves the system of equations using initial conditions and parameters to make predictions- With multiple subject data, this may be parallelized.
- With single subject the output data frame is adaptive
- Covariances and other metrics of uncertanty can be used to simulate while solving
Installation
You can install the released version of RxODE from CRAN with:
install.packages("RxODE")You can install the development version of RxODE with
devtools::install_github("nlmixrdevelopment/RxODE")To build models with RxODE, you need a working c compiler. To use parallel threaded solving in RxODE, this c compiler needs to support open-mp.
You can check to see if R has working c compiler you can check with:
## install.packages("pkgbuild")
pkgbuild::has_build_tools(debug = TRUE)If you do not have the toolchain, you can set it up as described by the platform information below:
Windows
In windows you may simply use installr to install rtools:
install.packages("installr")
library(installr)
install.rtools()Alternatively you can download and install rtools directly.
Mac OSX
To get the most speed you need OpenMP enabled and compile RxODE with that compiler. There are various options and the most up to date discussion about this is likely the data.table installation faq for MacOS. The last thing to keep in mind is that RxODE uses the code very similar to the original lsoda which requires the gfortran compiler to be setup as well as the OpenMP compilers.
If you are going to be using RxODE and nlmixr together and have an older mac computer, I would suggest trying the following:
If this crashes your R session then the binary does not work with your Mac machine. To be able to run nlmixr, you will need to compile this package manually. I will proceed assuming you have homebrew installed on your system.
On your system terminal you will need to install the dependencies to compile symengine:
After installing the dependencies, you need to reinstall symengine:
install.packages("symengine", type="source")
library(symengine)Linux
To install on linux make sure you install gcc (with openmp support) and gfortran using your distribution’s package manager.
Development Version
Since the development version of RxODE uses StanHeaders, you will need to make sure your compiler is setup to support C++14, as described in the rstan setup page. For R 4.0, I do not believe this requires modifying the windows toolchain any longer (so it is much easier to setup).
Once the C++ toolchain is setup appropriately, you can install the development version from GitHub with:
# install.packages("devtools")
devtools::install_github("nlmixrdevelopment/RxODE")Illustrated Example
The model equations can be specified through a text string, a model file or an R expression. Both differential and algebraic equations are permitted. Differential equations are specified by d/dt(var_name) =. Each equation can be separated by a semicolon.
To load RxODE package and compile the model:
library(RxODE)
#> RxODE 1.1.0 using 4 threads (see ?getRxThreads)
#> no cache: create with `rxCreateCache()`
mod1 <-RxODE({
C2 = centr/V2;
C3 = peri/V3;
d/dt(depot) =-KA*depot;
d/dt(centr) = KA*depot - CL*C2 - Q*C2 + Q*C3;
d/dt(peri) = Q*C2 - Q*C3;
d/dt(eff) = Kin - Kout*(1-C2/(EC50+C2))*eff;
})
#> Specify ODE parameters and initial conditions
Model parameters can be defined as named vectors. Names of parameters in the vector must be a superset of parameters in the ODE model, and the order of parameters within the vector is not important.
theta <-
c(KA=2.94E-01, CL=1.86E+01, V2=4.02E+01, # central
Q=1.05E+01, V3=2.97E+02, # peripheral
Kin=1, Kout=1, EC50=200) # effectsInitial conditions (ICs) can be defined through a vector as well. If the elements are not specified, the initial condition for the compartment is assumed to be zero.
inits <- c(eff=1);If you want to specify the initial conditions in the model you can add:
eff(0) = 1Specify Dosing and sampling in RxODE
RxODE provides a simple and very flexible way to specify dosing and sampling through functions that generate an event table. First, an empty event table is generated through the “eventTable()” function:
ev <- eventTable(amount.units='mg', time.units='hours')Next, use the add.dosing() and add.sampling() functions of the EventTable object to specify the dosing (amounts, frequency and/or times, etc.) and observation times at which to sample the state of the system. These functions can be called multiple times to specify more complex dosing or sampling regiments. Here, these functions are used to specify 10mg BID dosing for 5 days, followed by 20mg QD dosing for 5 days:
ev$add.dosing(dose=10000, nbr.doses=10, dosing.interval=12)
ev$add.dosing(dose=20000, nbr.doses=5, start.time=120,
dosing.interval=24)
ev$add.sampling(0:240)If you wish you can also do this with the mattigr pipe operator %>%
ev <- eventTable(amount.units="mg", time.units="hours") %>%
add.dosing(dose=10000, nbr.doses=10, dosing.interval=12) %>%
add.dosing(dose=20000, nbr.doses=5, start.time=120,
dosing.interval=24) %>%
add.sampling(0:240)The functions get.dosing() and get.sampling() can be used to retrieve information from the event table.
head(ev$get.dosing())
#> id low time high cmt amt rate ii addl evid ss dur
#> 1 1 NA 0 NA (default) 10000 0 12 9 1 0 0
#> 2 1 NA 120 NA (default) 20000 0 24 4 1 0 0
head(ev$get.sampling())
#> id low time high cmt amt rate ii addl evid ss dur
#> 1 1 NA 0 NA (obs) NA NA NA NA 0 NA NA
#> 2 1 NA 1 NA (obs) NA NA NA NA 0 NA NA
#> 3 1 NA 2 NA (obs) NA NA NA NA 0 NA NA
#> 4 1 NA 3 NA (obs) NA NA NA NA 0 NA NA
#> 5 1 NA 4 NA (obs) NA NA NA NA 0 NA NA
#> 6 1 NA 5 NA (obs) NA NA NA NA 0 NA NAYou may notice that these are similar to NONMEM event tables; If you are more familiar with NONMEM data and events you could use them directly with the event table function et
ev <- et(amountUnits="mg", timeUnits="hours") %>%
et(amt=10000, addl=9,ii=12,cmt="depot") %>%
et(time=120, amt=2000, addl=4, ii=14, cmt="depot") %>%
et(0:240) # Add sampling You can see from the above code, you can dose to the compartment named in the RxODE model. This slight deviation from NONMEM can reduce the need for compartment renumbering.
These events can also be combined and expanded (to multi-subject events and complex regimens) with rbind, c, seq, and rep. For more information about creating complex dosing regimens using RxODE see the RxODE events vignette.
Solving ODEs
The ODE can now be solved by calling the model object’s run or solve function. Simulation results for all variables in the model are stored in the output matrix x.
| time | C2 | C3 | depot | centr | peri | eff |
|---|---|---|---|---|---|---|
| 0 | 0.00000 | 0.0000000 | 10000.000 | 0.000 | 0.0000 | 1.000000 |
| 1 | 44.37555 | 0.9198298 | 7452.765 | 1783.897 | 273.1895 | 1.084664 |
| 2 | 54.88296 | 2.6729825 | 5554.370 | 2206.295 | 793.8758 | 1.180825 |
| 3 | 51.90343 | 4.4564927 | 4139.542 | 2086.518 | 1323.5783 | 1.228914 |
| 4 | 44.49738 | 5.9807076 | 3085.103 | 1788.795 | 1776.2702 | 1.234610 |
| 5 | 36.48434 | 7.1774981 | 2299.255 | 1466.670 | 2131.7169 | 1.214742 |
You can also solve this and create a RxODE data frame:
x <- mod1 %>% rxSolve(theta, ev, inits);
x
#> ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂ Solved RxODE object ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂
#> ── Parameters (x$params): ──────────────────────────────────────────────────────────────────────────────────────────────────────────────────────────────────
#> V2 V3 KA CL Q Kin Kout EC50
#> 40.200 297.000 0.294 18.600 10.500 1.000 1.000 200.000
#> ── Initial Conditions (x$inits): ───────────────────────────────────────────────────────────────────────────────────────────────────────────────────────────
#> depot centr peri eff
#> 0 0 0 1
#> ── First part of data (object): ────────────────────────────────────────────────────────────────────────────────────────────────────────────────────────────
#> # A tibble: 241 x 7
#> time C2 C3 depot centr peri eff
#> [h] <dbl> <dbl> <dbl> <dbl> <dbl> <dbl>
#> 1 0 0 0 10000 0 0 1
#> 2 1 44.4 0.920 7453. 1784. 273. 1.08
#> 3 2 54.9 2.67 5554. 2206. 794. 1.18
#> 4 3 51.9 4.46 4140. 2087. 1324. 1.23
#> 5 4 44.5 5.98 3085. 1789. 1776. 1.23
#> 6 5 36.5 7.18 2299. 1467. 2132. 1.21
#> # … with 235 more rows
#> ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂This returns a modified data frame. You can see the compartment values in the plot below:
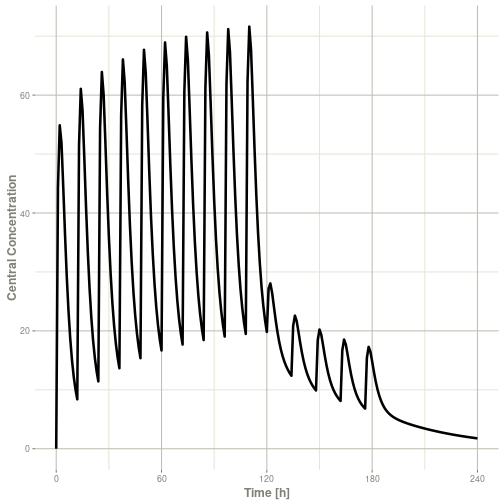
Or,
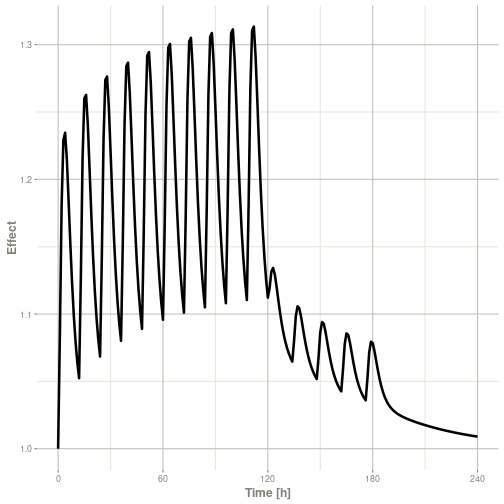
Note that the labels are automatically labeled with the units from the initial event table. RxODE extracts units to label the plot (if they are present).